An magnesium yana a yi amfani da shi a matsayin matar anoda a battery mai ukuwa saboda tasiri masu kyau. Wannan ita ce metal mai karfi. Kuma yana da shirya da take da shi saboda hanyar zuwa. Battery ta magnesium/manganese dioxide (Mg/MnO2) tana da tsari na biyu i.e. kadan da ya fi zinc/manganese dioxide (Zn/MnO2) battery da kadan da dama. Tana iya da shirya kadan har sai a lokacin da ke fara a cikin fushi, kuma har sai a faren dole. Battery ta magnesium tana da tsari da shirya saboda akwai abinci mai gaba da take da shi wanda yana zama babban jiki a kan surface ta magnesium anode.
Battery ta magnesium tana rasa shiryan da suke da shi idan an yi partial discharge, kuma wannan shine mafi yadda da ma ba a yi amfani da shi a cikin amfani mai tsari da biyu. Wannan shine mafi yadda da battery ta magnesium tana rasa shiryan, kuma battery ta lithium suna samu shi a kasuwar.
Kimiyar Battery ta Magnesium
A battery ta magnesium mai ukuwa, ana amfani da alloy ta magnesium a matsayin anoda; manganese dioxide ana amfani da shi a matsayin material ta cathode. Amma manganese dioxide bai iya bayar da shiga ga required conductivity ta cathode, kuma saboda haka acetylene black ana halatta da manganese dioxide don bayar da shiga ga required conductivity. Ana amfani da magnesium perchlorate a matsayin electrolyte. Barium da lithium chromate ana hada a electrolyte don in kare koroshi. Ana hada a mixture da magnesium hydroxide a matsayin buffering agent don in gina shirya.
Abubuwan da aka yi a anoda shine,


Abubuwan da aka yi a cathode shine,


Tushen abubuwan da aka yi,


Voltage ta open circuit, wannan cell tana bayar da volt 2 amma value na theoretical ta cell potential tana volt 2.8.
Tsari da magnesium yake da shi ita ce daidai hata a wurin fushi. Raw magnesium tana ci gaba da moisture kuma tana zama coating ta thin film ta Mg(OH)2 a kan surface.
Wannan thin film ta magnesium peroxide tana yi aikin da za a gaba da koroshi a kan magnesium. Saboda haka treatment ta chromate a kan magnesium tana gina daya aikin da za a gaba da koroshi. Amma idan wannan protective film ta magnesium peroxide tana punchered ko tana cika saboda discharge ta battery, koroshi tana faru da formation ta hydrogen gas.


Wannan shine kimiyar battery ta magnesium.
Construction of Magnesium Battery
Cylinder battery ta magnesium cell tana da resemblance da cylindrical zinc-carbon battery cell. A nan alloy ta magnesium ana amfani da shi a matsayin container na battery. Wannan alloy tana zama baki da magnesium da small quantity ta aluminum da zinc. A nan, manganese dioxide ana amfani da shi a matsayin material ta cathode. Saboda manganese dioxide tana da poor conductivity, acetylene black ana halatta da manganese dioxide don in gina shiga. Wannan tana taimakawa in da shirya water a kan cathode. A nan, barium chromate ana hada a cathode mix as inhibitor, kuma magnesium hydroxide ana hada a pH buffer. Magnesium perchlorate with lithium chromate mixed with water ana amfani da shi a matsayin electrolyte. Carbon tana saka a kan cathode mix as current collector. Kraft papers, absorbed with electrolyte solution tana saka a kan cathode da anoda materials as separators. Special attention tana bukatar a designing sealing arrangement a battery ta magnesium. Sealing ta battery bai iya zama porous da moisture a kan battery zai cika a lokacin da ke fara, kuma bai iya zama nonporous da hydrogen gas da ya faru a lokacin da ke discharge ba zai iya cika. Saboda haka, seal ta battery tana da shirya moisture a kan, kuma tana da vent da ya fi hydrogen gas da ya faru a lokacin da ke discharge. Wannan zai iya a yi da provision ta small hole a kan top ta plastic seal washed under the Retainer ring. Idan excess gas tana cika daga hole, retainer ring tana deforme saboda pressure kuma tana result a escaping from the gas.
a
Magnesium anode tana forma outer cover ta battery, amma construction na battery tana daɗi a cikin da carbon tana forma outer container ta battery. A nan, typically shaped container tana forma baki da highly conductive carbon. Wannan container tana forma a cylindrical cup shape, kuma one rod-like shape tana project from its center as shown in the picture. Anode ta battery tana forma baki da cylinder or drum ta magnesium. Diameter ta cylindrical anode tana da half da carbon cup. Cathode mix tana saka a kan anode cylinder kuma tana separate from inner wall ta cylinder by paper separator. Space a kan inner surface ta carbon cup da outer surface ta anode cylinder tana filled with cathode mix kuma tana separate from cathode mix by paper separator. Cathode mix tana produce baki da mixing manganese dioxide, carbon black, and small quantity of aqueous magnesium bromide or perchlorate as the electrolyte. Positive terminal tana connect to end of carbon cup. Negative terminal tana connect to end of anode drum. Entire system tana encapsulated in crimped tin-plated steel jacket.
Advantage of Magnesium Battery
It has a very good self life; it can be stored for a long time even under high-temperature. These battery can be stored up to 5 years at the temperature 20oC.
It has twice capacity compared to equivalent size Leclanche battery.
Higher battery voltage than zinc-carbon battery.
Cost is also moderate.
Disadvantages of Magnesium Battery
Delayed action.(voltage delay)
Evolution of hydrogen during discharge.
Heat generated during use.
Poor storage after partial discharge.
The battery are no longer manufactured commercially.
Sizes And Types Of Mg/MnO2 Batteries
Cylindrical Magnesium Primary Batteries
| Battery type |
Diameter in mm |
Height in mm |
Weight in gm |
Capacity in Ah |
| N |
11 |
31 |
5 |
0.5 |
| B |
19.2 |
53 |
26.5 |
2 |
| C |
25.4 |
49.7 |
45 |
3 |
| 1LM |
22.8 |
84.2 |
59 |
4.5 |
| D |
33.6 |
Ba da kyau kuma kara mai rubutu!
Tattalin da Gudummawa da Turanci na Noma Kirkiyya
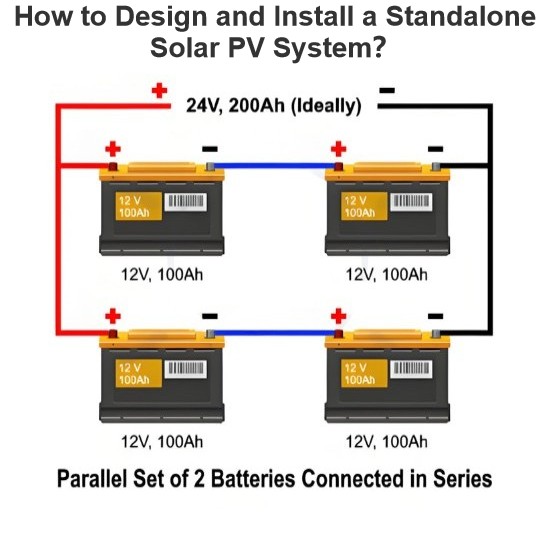
Gurbin Da Iya Karya Da Photovoltaic (PV) Na NomaTattalin noma na photovoltaic (PV) yana da muhimmanci mai PV, kontrola, inbirta, batari, da wasu abubuwa masu tashin (batari ba zan iya bukata don tattalin noma na grid). Idan kuna neman cewa an yi amfani da shirye-shiryar gwamnati, ana gaba tattalin noma na PV zuwa wata na off-grid da wata na grid-connected. Tattalin noma na off-grid ke kusa da suka yi aiki biliyan-biliyan baya bayan shirye-shiryar gwamnati. Suna da batari don inganta kyauwar taka
Ko kuke so ku yi aiki na ƙaramin PV Plant? State Grid Yawanci Amsa 8 Tattalin Yawancin O&M (2)
1. A ranar na rana mai karfi, ya kamfanon da suka lalace da ake kare da shi suka fi zama da wuya?Ba a taka tabbacin da za a yi gaba ba. Idan an bukata da tabbacin, yana da kyau a yi shi a ranar na baya ko kuma a ranar na gaskiya. Zaka iya tuntubi masu mulki na birnin kuraci (O&M) kuma bayan samun malaman da za su iya zuwa wurin don yi tabbacin.2. Don in hana PV modules daga inganta abubuwa mai tsawo, ana iya sanya sabbin jirgin da ke cikin PV arrays?Ba a taka sanya sabbin jirgin ba. Wannan i
Ko Da Daidaituwa Masana'antu PV? State Grid Ya Bayar 8 Taswirin O&M Mafi Yawan Gudanar (1)
1. Na wani abubuwa da aka fi sani a cikin yanayi masu yawan gida na karkashin zafi (PV) suna da shi? Wadannan muhimman abubuwa masu iya faru a cikin farkon tushen yanayin?Muhimman abubuwan da suka faru sun hada da inverter bai yi aiki ko kuma bai faru ba saboda tsari ba ta fadada darajar da ake kafa, da kuma yawan gida mai kadan da ya faru saboda matsalolin PV modules ko inverters. Muhimman abubuwan da za su iya faru a cikin farkon tushen yanayin sun hada da kisan junction boxes da kuma kisan PV
Ko kari da Kowa da Sabon Kirkiya na Solar PV?
Solar PV Systems na Kowa da Kudin KasaAl'adu mai karfi ta harkar zafi suna amfani da kuli masu yawan adawa, kamar tattalin arziki, kula, yanayi, da kuma noma, musamman daga abubuwa mai ba da rikitar (kula, gida, gas). Amma, waɗannan suna ƙara ƙwace-gabashin al'umma, suka fito, da kuma samun ci gaba ɗaya ga tsawon sama. Saboda haka, ana bukatar tabbataccen jirgin kuli.Kuli mai zurfi, wanda ya fi shi da kuli da za a iya koyar muhimmancin adawa, ya ƙare. Muhimmiyar PV systems (Fig 1) suna bayar da
Samun IEE Business Application
Yi amfani da IEE-Business app don samun abubuwan aikin, samun halayyin, haɗi da malamai, kuma kai tsauraran takaiddun kasoshin duka lokaci, duka wurin—dole bai karfin takamaltar hulɗin ku na alintakargida da kasuwanci.
|