Since the second half of the 19th century, the only insulating materials suitable for high-voltage power transmission lines were ceramics and glass. Starting in the 1940s, with the emergence of polymer materials, ceramics and glass were no longer the preferred choices, prompting countries in Europe and America to begin researching polymer insulators. Subsequently, extensive studies were conducted on the physical properties, electrical characteristics, long-term reliability, and optimal shapes of electrical insulators, and production efficiency continued to improve.
Among high-molecular-weight materials capable of replacing ceramics and glass, silicone rubber has demonstrated practical application performance since the 1960s and has stood out among various polymers. Silicone rubber insulators offer several advantages over ceramic insulators: first, they are lightweight, easy to handle, and safer; second, ceramic insulators are prone to cracking under impact, whereas silicone rubber insulators can effectively withstand mechanical shocks such as vehicle collisions with utility poles.
Although other polymer materials also possess the aforementioned advantages, only silicone rubber causes minimal environmental pollution. Polymer insulators are water-resistant, preventing leakage current and surface arcing caused by water droplets. Moreover, the hydrophobicity of silicone rubber insulators recovers faster than that of other polymer insulators, making them a durable material suitable for long-term use in harsh environments. This article explains the characteristics of silicone rubber used in high-voltage electrical insulation and introduces recent development trends.
1 Characteristics of Silicone Rubber
1.1 Chemical Characteristics of the Siloxane Bond
1.1.1 Chemically Stable Bond
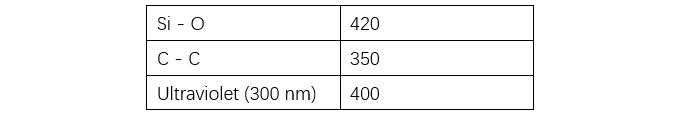
The backbone of silicone rubber consists of siloxane (Si-O) bonds. Due to the significant difference in electronegativity between Si (1.8) and O (3.5), a polarized structure is formed, as shown in Figure 1 (omitted), exhibiting ionic bond characteristics. Consequently, the bond energy of Si-O is higher than that of C-C (see Table 1). Furthermore: (1) due to the ionic nature of the main chain, the polarity of the methyl C-H groups in the side chains is reduced, making them less susceptible to attack by other molecules, thus resulting in excellent chemical stability; (2) since Si does not readily form double or triple bonds, the main chain is less prone to decomposition, and Si-C bonds are consequently very stable, further enhancing the stability of the silicone rubber backbone.
1.1.2 High Flexibility Polymer
The bond angle of siloxane (Si-O-Si) is large (130°–160°), giving it higher freedom than organic polymers (C-C bond angle ~110°). Additionally, the Si-O bond length (1.64 Å) is longer than that of C-C (1.5 Å). This means the overall polymer molecule is more mobile and easier to deform.
1.1.3 Helical Structure
Due to the helical structure of polysiloxane, the siloxane bonds on the main chain are drawn inward by ionic attraction, while the outer side consists of methyl groups with weak intermolecular interactions, resulting in weak intermolecular forces.
1.2 Properties of Silicone Rubber
Based on the chemical characteristics described in Section 1.1, silicone rubber possesses the following properties suitable for high-voltage electrical insulation.
1.2.1 Heat and Cold Resistance
Due to its high bond energy and excellent chemical stability, silicone rubber has better heat resistance than organic polymers. Additionally, because of weak intermolecular forces, it has a low glass transition temperature and excellent cold resistance. Therefore, its performance remains stable regardless of the geographic region in which it is used.
1.2.2 Water Resistance
The surface of polysiloxane is composed of methyl groups, giving it hydrophobic properties and thus excellent water resistance.
1.2.3 Electrical Properties
Silicone rubber contains fewer carbon atoms than organic polymers, resulting in excellent arc resistance and tracking resistance. Moreover, even when burned, it forms insulating silica, further ensuring superior electrical insulation performance.
1.2.4 Weather Resistance
As shown in Table 1, the bond energy of siloxane is higher than the energy of ultraviolet (UV) light, making it resistant to UV-induced aging. In accelerated ozone resistance tests, organic polymers crack within seconds to hours, whereas silicone rubber shows only a slight reduction in strength after four weeks of aging, with no cracking observed, indicating excellent ozone resistance (see Table 2). Acid rain is a mixed ionic solution with a pH of approximately 5.6. A 500x concentrated artificial acid rain test was conducted using the solution listed in Table 3. Silicone rubber exhibits excellent chemical resistance as shown in Table 4. Although exposure to mixed solutions like acid rain may cause some changes, the impact is expected to be minimal.
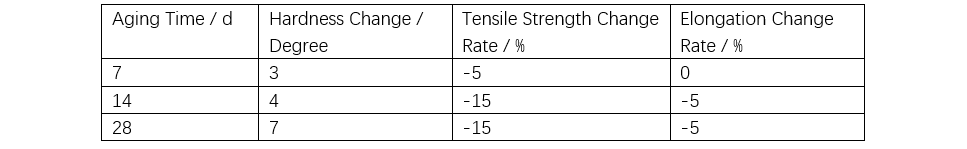
Note: At room temperature, with an ozone concentration of 200 ppm and a 50% tensile strain applied to the rubber, the surface shows no cracking even after 28 days of aging.
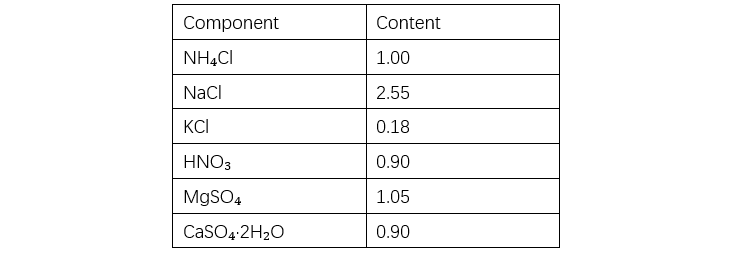
Unit: g per 2 L of deionized water.
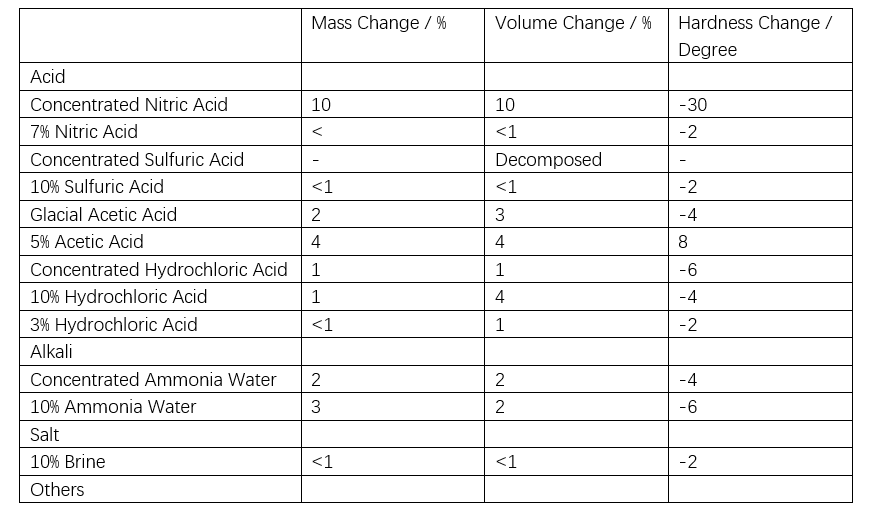
1.2.5 Permanent Deformation
Silicone rubber exhibits better permanent deformation characteristics (including permanent elongation and compression set) at both room and elevated temperatures compared to organic polymers.
2 Classification of Silicone Rubber
Silicone rubber can be classified into solid and liquid types based on its state prior to vulcanization, and into peroxide curing, addition curing, and condensation curing types based on the vulcanization mechanism. The main difference between solid and liquid silicone rubber lies in the molecular weight of the polysiloxane. Solid silicone rubber can be vulcanized via either peroxide curing or addition curing, and is commonly referred to as high-temperature vulcanizing rubber (HTV) or heat-cured rubber (HCR) (see Tables 5 and 6).
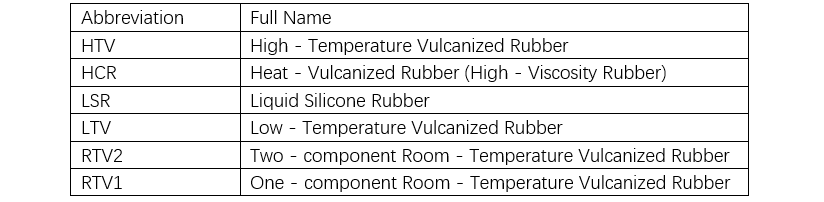
Although liquid silicone rubber cured by addition reaction can also vulcanize at room temperature, it is designated as liquid silicone rubber (LSR), low-temperature vulcanizing rubber (LTV), or two-part room-temperature vulcanizing rubber (RTV), depending on the processing method and curing temperature. In the manufacturing of polymer insulators, injection molding and casting are commonly used processes.
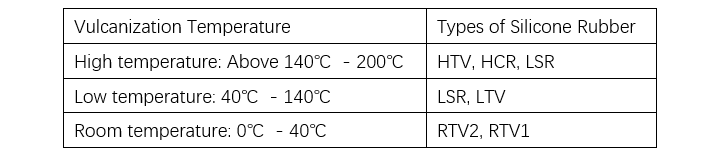
One-component condensation-type (moisture-cure) silicone rubber can be used in construction sealants, as well as in electrical and electronic products. In electrical applications, solvent-diluted room-temperature vulcanizing (RTV) silicone rubber coatings are commonly sprayed onto ceramic insulators as protective materials.
2.1 Silicone Rubber with Aluminum Trihydroxide (ATH)
Silicone rubber with good tracking resistance and arc resistance can be obtained by incorporating a high loading of aluminum trihydroxide (ATH). Silicone rubber filled with 50 parts by mass of ATH exhibits acceptable resistance to high-voltage (4.5 kV) tracking, along with excellent arc resistance, weather resistance, salt fog resistance, and acid rain resistance, making it suitable as an insulating material in areas with severe salt fog. However, due to the high ATH loading, this material suffers from high viscosity (poor plasticity) and low mechanical strength.
2.2 Silicone Rubber without Aluminum Trihydroxide (ATH)
In inland areas of Europe and similar regions with minimal salt fog and low pollution levels, silicone rubber without ATH filler can be used. In such cases, appropriate selection of the base silicone rubber, surface treatment of fumed silica, and addition of compounding agents that enhance tracking resistance can improve hydrophobicity to meet high-voltage tracking resistance requirements. Compared to ATH-filled silicone rubber, this type has lower viscosity and superior mechanical and electrical properties.
2.3 For Outdoor Cable Accessories
As outdoor cable accessories are exposed to harsh environments, they must possess good tracking resistance. Materials with low permanent elongation can be achieved by using polymers with optimized crosslinking density, suitable for ambient-temperature shrinkable (cold-shrink) products.
2.4 For Indoor Cable Accessories
Indoor cable accessories are unlikely to be affected by salt fog, so tracking resistance is often not required. However, when used in ambient-temperature shrinkable (cold-shrink) applications, low permanent deformation characteristics are still necessary.
2.5 Coating Applications
Spraying silicone rubber coatings onto heavily polluted areas can maintain good hydrophobicity over the long term. Coatings can also be applied to already-installed insulators based on pollution levels, enabling continued service and cost savings. Recent reports indicate that coating silicone rubber insulators can further enhance the retention of hydrophobicity. Currently, two main types exist: coated insulators and rubber-type insulators.
3 Conclusion
This paper has introduced silicone rubber materials for polymer insulators. Ongoing research and testing are being conducted by various institutions and manufacturers. If high reliability can be demonstrated through durability and other performance tests, the application of silicone rubber insulators is expected to expand further.