Mai Suna Mata Valence Electrons da Electrical Conductivity?
Takardunin Valence Electrons
Aikatau mai yawan atom da na nukilus mai girman proton da neutron, kuma electrons a wurare na. Nukilus yana ciki da mafi karfi, kuma electrons yana ciki da mafi yau. Atoms suna da tsari saboda suka da adadin proton da electrons da dama.
Electrons a atom an sauka da wurare da kyau basu masu energy levels. Wuraren da ya fi nukilus yana da mafi yawan energy, kuma wuraren da ya fiya yana da mafi karfin energy. Har wura na iya gudanar da electrons: wuran farko yana iya gudanar da 2, wuran biyu yana iya gudanar da 8, kuma baya haka.
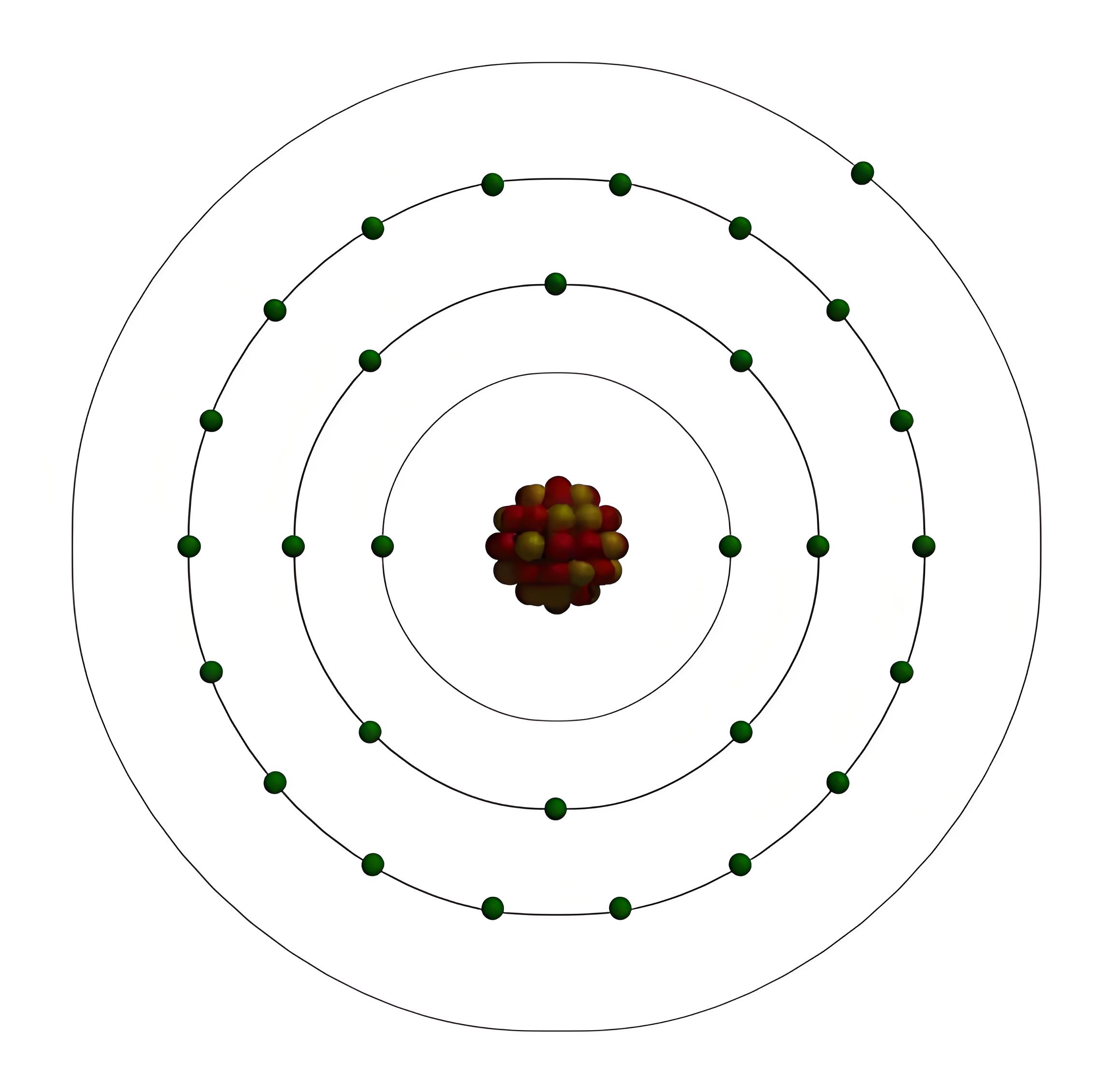
Valence electrons suna cewa electrons a wuraren da ya fi a atoms. Suke take part a chemical bonding kuma zai iya kasancewa electric fields ko magnetic fields. Adadin valence electrons yana haɗa da 1 zuwa 8, kafin element.
Valence electrons suna da muhimmanci a kan tsarin physical, chemical, da electrical properties ta element. Elements da suka da valence electrons da dama suna da reactivity da bonding types da dama. Adadin valence electrons da ke goma yana ba da electrical conductivities da material types da ke goma.
Electrical Conductivity
Electrical conductivity tana ce koyar da matsayin yadda material yake iya lashe electric current a fadada. Electric current tana shahara da electric charges da suka yi lalle, musamman da free electrons ko ions. Materials da mafi electrical conductivity suna iya lashe current da dangane, amma materials da mafi resistance suna iya ciwo.
Electrical conductivity ta material tana baki da abubuwa da suka haɗa da temperature, structure, composition, da purity. Amma, babban abubuwan da ke muhimmanci shine adadin da behavior of free electrons a material.
Free electrons suna cewa valence electrons da ba su da takalma a parent atoms su kuma zai iya yi lalle a material. Waɗannan electrons suna iya jin daɗi applied electric field ko potential difference kuma zai iya yi lalle a fadada, don bayyana electric current.
Adadin da behavior of free electrons a material tana baki da adadin valence electrons a constituent atoms. Ba ɗaya, materials da mafi valence electrons suna da mafi free electrons, amma materials da mafi valence electrons suna da mafi free electrons.
Daga baya, materials zai iya kula da kungiyoyi uku: conductors, semiconductors, da insulators.
Conductors
Conductors suna cewa materials da suka da mafi electrical conductivity saboda suka da mafi free electrons da zai iya lashe electric current da dangane. Conductors suna da one, two, ko three valence electrons a atoms. Waɗannan valence electrons suna da mafi energy levels kuma suna da takalma da mafi yawa a parent atoms. Zai iya fitowa daga atoms ko yi lalle a material idan applied electric field ko potential difference.
Yawan metals suna da mafi electrical conductivity saboda suka da mafi valence electrons a atoms. Misali, copper yana da one valence electron, magnesium yana da two valence electrons, kuma aluminum yana da three valence electrons. Waɗannan metals suna da mafi free electrons a crystal structure su da zai iya yi lalle idan applied electric field.
Abubuwa nonmetals suna iya yi waɗanda conductors a wasu halayen. Misali, graphite (form of carbon) yana da four valence electrons a atoms, amma only three of them yana da bonding with other carbon atoms a hexagonal lattice. The fourth valence electron yana iya yi lalle a lattice idan applied electric field.
Semiconductors
Semiconductors suna cewa materials da suka da electrical conductivity da yawa saboda suka da mafi free electrons da zai iya lashe electric current a wasu halayen. Semiconductors suna da four valence electrons a atoms, misali, carbon, silicon, da germanium. Waɗannan valence electrons suna da bonding with other atoms a regular lattice structure. Amma, a room temperature, some of these valence electrons zai iya samun mafi energy don fitowa daga bonds su kuma zai iya yi lalle a material. Waɗannan free electrons zai iya lashe electric current idan applied electric field.
Amma, adadin free electrons a pure semiconductor yana da mafi yawa, kuma electrical conductivity yana da mafi yawa. Saboda haka, semiconductors suna daidaita da impurity atoms da suka da mafi ko mafi valence electrons than the host atoms. Wannan yana ba da excess or a deficiency of free electrons in the semiconductor, kuma yana zama electrical conductivity.
An kula doping da n-type and p-type. A n-type doping, impurity atoms da suka da five valence electrons, misali, phosphorus ko arsenic, suna daidaita a semiconductor. Waɗannan atoms suna ba da extra valence electron to the semiconductor, creating a negative charge carrier called an electron. A p-type doping, impurity atoms da suka da three valence electrons, misali, boron ko gallium, suna daidaita a semiconductor. Waɗannan atoms suna accept one valence electron from the semiconductor, creating a positive charge carrier called a hole.
Semiconductors suna amfani da su a yawan electronic devices, misali, transistors, diodes, solar cells, light-emitting diodes (LEDs), lasers, da integrated circuits. Waɗannan devices suna amfani da unique properties of semiconductors, misali, ability to switch between conducting and insulating states, sensitivity to light and temperature, da compatibility with other materials.
Insulators
Insulators suna cewa materials da suka da mafi electrical conductivity saboda suka da mafi free electrons da zai iya lashe electric current. Insulators suna da five or more valence electrons a atoms. Waɗannan valence electrons suna da takalma da mafi yawa a parent atoms kuma ana buƙaci mafi energy don fitowa. Saboda haka, insulators ba su iya jin daɗi applied electric field ko potential difference kuma suke ciwo da flow of electric current.
Yawan nonmetals suna da mafi electrical conductivity saboda suka da mafi valence electrons a atoms. Misali, nitrogen yana da five valence electrons, sulfur yana da six valence electrons, kuma neon yana da eight valence electrons. Waɗannan elements suna da mafi free electrons a structure su kuma ba su iya lashe electric current a fadada.
Abubuwa materials suna iya yi waɗanda insulators a wasu halayen. Misali, glass and rubber suna da mafi electrical conductivity a room temperature amma zai iya yi waɗanda conductors a high temperatures idan some of their valence electrons samu mafi energy don fitowa daga atoms su.
Insulators suna amfani da su don ciwo electric current a wajen da ba a yi da ita ko da ita. Misali, insulators suna amfani da su don coat wires and cables don protect them from short circuits and electric shocks. Insulators suna amfani da su don separate different parts of an electronic device or circuit don ciwo unwanted interactions or interference.
Kawo Kammal
Valence electrons suna cewa electrons a wuraren da ya fi a atom da zai iya take part a chemical bonding and electrical current. Adadin da arrangement of valence electrons tana baki da yawan physical, chemical, da electrical properties ta element.
Electrical conductivity tana ce koyar da matsayin yadda material yake iya lashe electric current. Electrical conductivity tana baki da abubuwa, misali, adadin da behavior of free electrons a material.
Daga baya, materials zai iya kula da kungiyoyi uku: conductors, semiconductors, da insulators.
Conductors suna da mafi electrical conductivity saboda suka da mafi free electrons da zai iya lashe electric current. Conductors suna da one, two, ko three valence electrons a atoms.
Semiconductors suna da electrical conductivity da yawa saboda suka da mafi free electrons da zai iya lashe electric current a wasu halayen. Semiconductors suna da four valence electrons a atoms.
Insulators suna da mafi electrical conductivity saboda suka da mafi free electrons da zai iya lashe electric current. Insulators suna da five or more valence electrons a atoms.
Waɗannan materials suna amfani da su a yawan electronic devices, misali, transistors, diodes, solar cells, LEDs, lasers, da integrated circuits. Waɗannan devices suna amfani da unique properties of waɗannan materials, misali, ability to switch between conducting and insulating states, sensitivity to light and temperature, da compatibility with other materials.